The human brain stands apart in the animal kingdom, not just in sheer size but in its remarkable cognitive abilities. For decades, researchers have sought to understand what genetic changes fueled the expansion of our neocortex, the region responsible for higher-order thinking, reasoning, and language. A recent study published in Science Advances1 by Nesil Eşiyok and colleagues sheds light on this question, identifying a crucial genetic duo—NBPF14 and NOTCH2NLB—that orchestrates the abundance of neural progenitor cells, a key factor in the evolutionary enlargement of the human brain.
The Role of NBPF14 and NOTCH2NLB
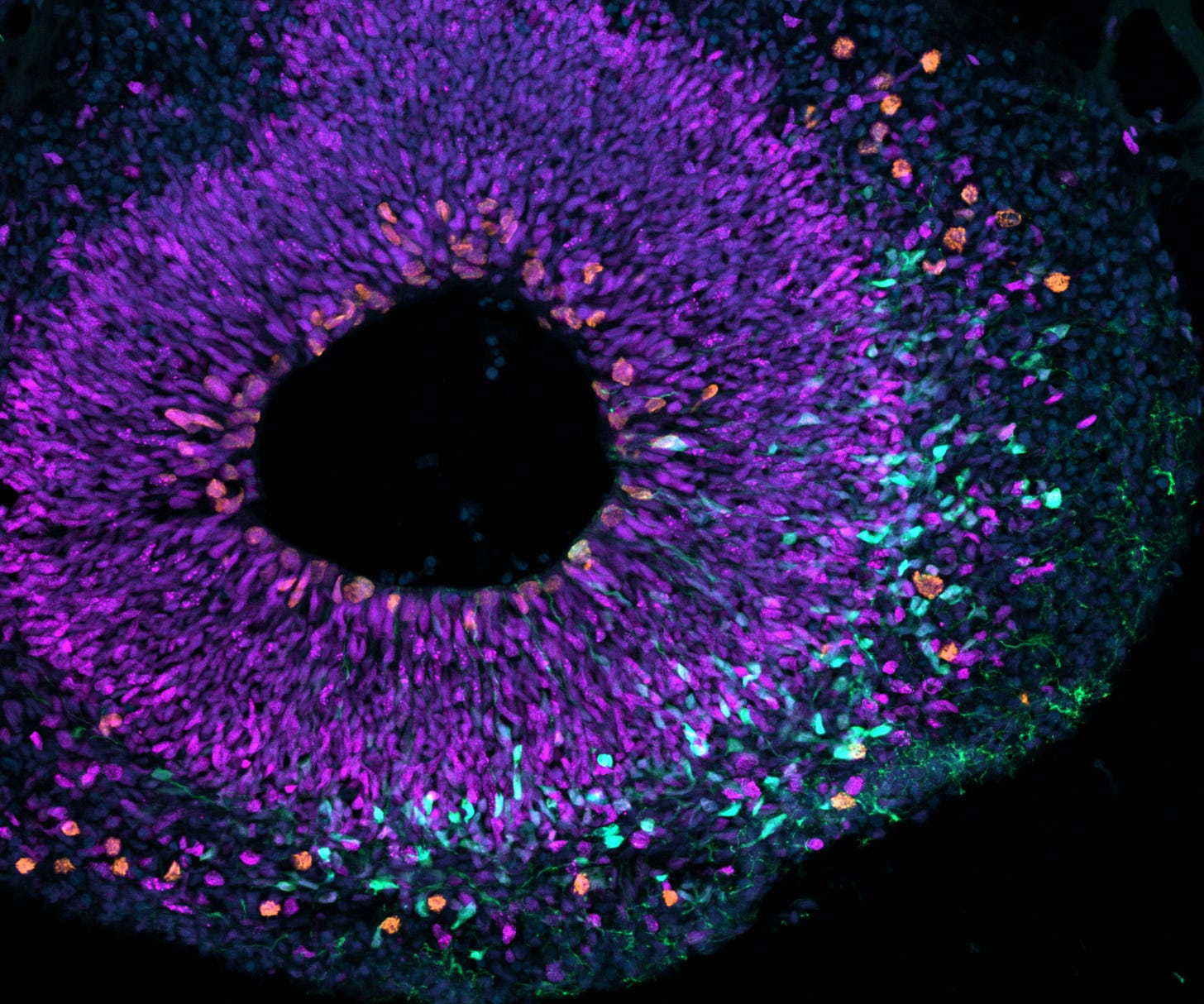
At the heart of this discovery is the interplay between two genes that are uniquely human. The study demonstrates that NBPF14 and NOTCH2NLB function in tandem to regulate the proliferation and transformation of neural progenitor cells, the building blocks of the developing neocortex.
NBPF14 stimulates the multiplication of these progenitor cells, ensuring an ample supply during brain development.
NOTCH2NLB directs these progenitor cells toward a more specialized type, which eventually matures into the neurons that compose our brain’s intricate networks.
This genetic interaction sets humans apart from other primates, providing a biological explanation for why our neocortex is uniquely large and complex.
Experimental Evidence: A Multi-Faceted Approach
To uncover the role of these genes, researchers combined multiple experimental approaches, including:
Mouse Models: Introducing human versions of these genes into mice resulted in an increase in neural progenitor cells, suggesting a direct link between gene function and brain expansion.
Chimpanzee Brain Organoids: By cultivating miniature brain-like structures from chimpanzee stem cells, the researchers observed how the absence of these human-specific genes limited neural progenitor proliferation.
Human Brain Tissue Analysis: Further supporting their findings, the study examined human fetal brain tissue to confirm the expression patterns of NBPF14 and NOTCH2NLB in early brain development.
"The remarkable feature of our study is that results from both animal models and alternative methods complement each other well and mutually confirm their findings," explains Michael Heide, the study’s lead researcher. "This not only underscores the significance of our work but also highlights the potential for reducing reliance on animal models by refining alternative methods."
Evolutionary and Medical Implications
Understanding the genetic underpinnings of human brain expansion has profound implications beyond evolutionary biology. These insights may help illuminate the origins of neurodevelopmental disorders such as microcephaly, in which brain size is significantly reduced due to disruptions in progenitor cell proliferation.
"Our findings deepen the fundamental understanding of brain development and provide new insights into the evolutionary origins of our large brain," says Nesil Eşiyok, first author of the study. "In the long term, they could contribute to the development of therapeutic approaches for malformations of the brain."
The Future of Human Brain Evolution Research
This study adds another piece to the puzzle of human brain evolution, but many questions remain. How did these genes originate and become unique to humans? What other genetic or environmental factors contributed to the increase in brain size over millions of years? Future research will continue to probe these mysteries, combining genetic analysis with fossil and archaeological evidence to paint a more complete picture of our evolutionary past.
With each discovery, the story of human brain evolution becomes clearer, offering a deeper appreciation for the genetic symphony that shaped our species’ most defining trait—our intelligence.
Eşiyok, N., Liutikaite, N., Haffner, C., Peters, J., Heide, S., Oegema, C. E., Huttner, W. B., & Heide, M. (2025). A dyad of human-specific NBPF14 and NOTCH2NLB orchestrates cortical progenitor abundance crucial for human neocortex expansion. Science Advances, 11(13). https://doi.org/10.1126/sciadv.ads7543








Share this post